| 〔Case Report〕 |
Gastric GIST and sigmoid colon neurofibroma in a
patient of neurofibromatosis type 1 |
|
Mitutune Washiro1,2), Kenji Oda2), Hirokazu Oshima1), Toshio Omori1)
Yuji Himeno1), Kazuhiro Seike2), Keiji Koda3) and Masaru Miyazaki2) |
| (Received February 2, 2006, Accepted February 24, 2006) |
|
SUMMARY
Gastrointestinal stromal tumor (GIST) of stomach in a neurofibromatosis type 1 (NF1) patient is very rare. We report herein a case of 61-year-old woman of NF1 with gastric GIST and sigmoid colon neurofibroma. Preoperative examinations revealed a gastric submucosal tumor measuring 4 cm on the body of the stomach and an intramural 2.5 cm sized submucosal tumor at the sigmoid colon. Gastric wedge resection and partial sigmoid colon resection were performed. Immunohistochemically, gastric tumor was diagnosed as a GIST, uncommitted type, and colon lesion was diagnosed as a neurofibroma. Further accumulation and molecular analysis of similar cases will be necessary to elucidate the association of NF1 with GISTs.
|
|
Key Words
| neurofibromatosis type 1, gastric GIST, neurofibroma, c-kit, platelet-derived growth factor receptor alpha |
|
| |
I. Introduction
Gastrointestinal involvement in von Recklinghausen’s disease or neurofibromatosis type 1 (NF1) has been reported to occur in 11 % to 25 % of cases, and such lesions had been considered to have neurogenic origin[1,2]. However, advance of molecular biology and immunohistochemical study has introduced the histologically neutral term, gastrointestinal stromal tumor (GIST) . Some authors reported GISTs in NF1 patients, but the association of these entities was not clearly elucidated[3-7]. Recently, genetic analysis has been conducted to examine KIT and platelet-derived growth factor receptor alpha (PDGFRA) mutation status in NF1-associated GISTs[8-10]. In this article, we present a rare case of solitary GIST of the stomach and a neurofibroma of the sigmoid colon in a patient of NF1, and describe the microscopic, immunohistochemical features of these lesions.
II. Case
A 61-year-old Japanese woman was hospitalized for the evaluation of palpitation, dyspnea and tarry stool of a few days duration. The cutaneous stigmata of neurofibromatosis were present, and there were various caf-au-lait spots on the body. Histological examination revealed that these skin nodules were neurofibromas. Upper gastrointestinal series showed an intramural 4 x 4 cm sized submucosal mass at the body of the stomach. On gastroendoscopy, the tumor surface was irregularly eroded. Computed tomography (CT) revealed a slightly enhanced mass with a remarkable intraluminal growth. An intramural 2.5 x 2.5 cm sized submucosal tumor was also found at the sigmoid colon by colonoendoscopic examination.
Exploratory laparotomy was undertaken and gastric wedge resection and partial sigmoid colon resection were performed.
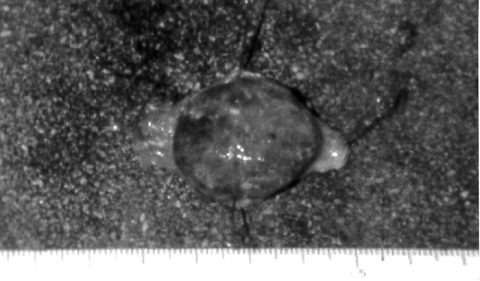
The stomach lesion was 4 x 4 x 2 cm in size, and the tumor surface was irregular with hemorrhagic areas (Fig. 1) . Histologically, the tumor cells were composed of spindle cells. The nuclei of tumor cells were slightly pleomorphic but mitosis was not frequently observed. Immunohistochemically, the tumor cells were positive for c-kit (CD117) and CD34 (Fig. 2) , but were negative for myogenic marker (α-SMA) and for neurogenic marker (S-100 protein) . The lesion was diagnosed as a GIST, uncommitted type.
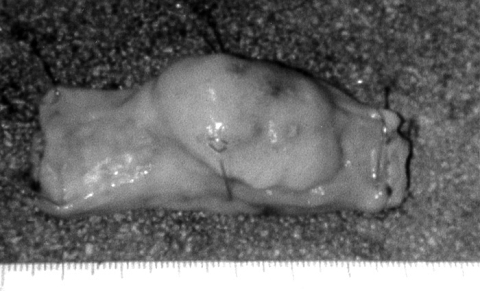
The colon lesion, measuring 4 x 2 x 2 cm, had gray, smooth surface (Fig. 3) . On light microscopy, the border of the tumor was unclear, and the tumor cells were composed of spindle cells with palisading pattern indicating neurogenic tumor. Mitotic figures were not observed. Immunohistochemically, the tumor cells were negative for c-kit, CD34, α-SMA, but positive for S-100 protein (Fig. 4) . The lesion was diagnosed as a neurofibroma.
The postoperative course of the patient was uneventful, and 4 years later, she was well and had no sign of recurrence.
|
 |
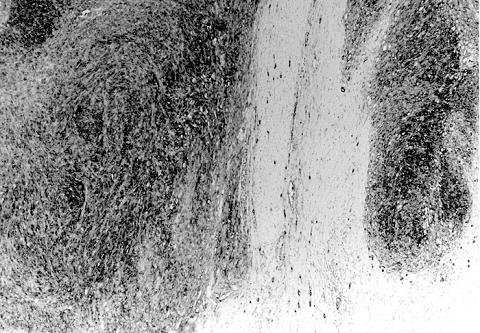 |
Fig. 1
A macroscopic view of the gastric tumor showing intraluminal growth and irregular, hemorrhagic surface. |
Fig. 2
Microscopy of the gastric lesion: spindle-shaped tumor cells immunoreactive to CD34 with clear margin. Original magnification: 80x.
|
|
 |
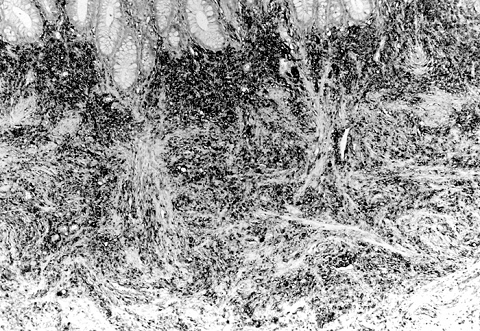 |
Fig. 3
A macroscopic view of the sigmoid colon tumor showing intraluminal growth and smooth surface. |
Fig. 4
Microscopy of the sigmoid colon lesion: spindle-shaped tumor cells immunoreactive to S-100 protein with unclear margin. Original magnification: 90x. |
|
III. Discussion
Neurofibromatosis, first clearly described by von Recklinghausen, is a dominantly inherited neuroectodermal dysplasia. However, 40 % to 50 % of the patients are reported to be sporadic cases. It is one of the most common genetic disorders, affecting one in 3000 births, and involving males slightly more often than females[2]. Characteristic lesions include caf-au-lait spots and neurofibromas along peripheral nerves[2].
Identification of the neurofibromatosis type 1 (NF1) gene was conducted in 1990. The NF1 gene product, neurofibromin, contains a GTPase-activating protein (GAP) -related domain, that is able to down-regulate p21ras by stimulating its intrinsic GTPase[11]. The GAP activity of the mutant NF1 gene product is reported to be 200- to 400-fold lower than that of wild type, whereas binding affinity is unaffected[12].
Gastrointestinal involvement occurs in as many as 25 percent of cases, and neurofibromas occur most frequently in the stomach and jejunum, but the colon also may be involved[13]. Gastrointestinal neurofibromas may cause occult bleeding, constipation, and obstruction[1,2,13].
Diagnostic advance of immunohistochemistry led to the introduction of the histogenetically neutral term of gastrointestinal stromal tumor (GIST) . Some authors reported the GISTs in the patients of neurofibromatosis type 1 (von Recklinghausen’s disease) , and suggested the possible association of these two disease entities[3-7]. In 1984, Schaldenbrand and Appelman[3]mentioned the two cases of GISTs of the jejunum and duodenum in NF1 patients with minimal smooth muscle differentiation. Mori et al[6]reported the 20 cases of the small intestinal GISTs accompanied with NF1 and mentioned the clinicopathological features. In 2002, Giuly et al[7]reported the malignant GIST of the duodenum in NF1 patient. From these reports, GISTs in NF1 patients seems to be most common in the small intestine and duodenum, and the most frequent symptom of GIST in NF1 is gastrointestinal bleeding. In the present case, the gastric tumor was diagnosed as GIST, uncommitted type. In our knowledge, the gastric GIST in NF1 is very rare.
Recently, genetic analysis has been conducted to examine c-kit and PDGFRA mutations in NF1-associated GISTs[8-10]. The authors concluded that the mutations of c-kit and PDGFRA gene might play a limited role in the tumorigenesis of NF1-associated GISTs. In contrast to sporadic GIST, NF1-associated GISTs are unique with regard to younger onset, multicentricity, predominant location in the small bowel, spindle cell morphology, CD34 immunoexpression, lack of c-kit and PDGFRA mutations and more favorable clinical course.
In conclusion, we report a rare case of NF1 with gastric GIST and sigmoid colon neurofibroma. Further accumulation of reports of similar cases and development of immunohistochemical and molecular studies will be necessary to elucidate this association. |
|
要旨
神経線維腫症1型に胃GISTを合併することは稀である。今回我々は胃GISTおよびS状結腸神経線維腫を併発した神経線維腫症1型を経験したので報告する。症例は61歳女性で動悸,息切れ,下血を主訴に来院した。皮膚には神経線維腫症およびカフェオレ班が観察された。術前検査では胃体部にびらんを伴う4㎝大の粘膜下腫瘍,S状結腸に2.5㎝大の粘膜下腫瘍が認められた。胃楔状切除およびS状結腸部分切除術が施行された。病理学的検索では,胃病変はc-kit, CD34陽性のGIST, uncommitted typeであり,S状結腸病変はS-100蛋白陽性の神経線維腫であった。術後経過は良好で4年経過後の現在でも再発の徴候は認められていない。文献的考察では,神経線維腫症1型に伴うGISTでは散発性GISTと比較し,c-kit, PDGFRAの突然変異を検出する頻度は低く,若年発症,多中心性,小腸優位の発生,紡錘型の細胞形態,CD34発現頻度の増加,予後良好などの特徴を有している。
|
|
References
1) Davis GB, Berk RN. Intestinal neurofibromas in von Recklinghausen’s disease. Am J Gastroenterol 1973; 60: 410-5.[PubMed]
2) Hochberg FH, Dasilva AB, Galdavini J, Richardson EP. Gastrointestinal involvement in von Recklinghausen’s neurofibromatosis. Neurology 1974; 24; 1144-51.
3) Schaldenbrand JD, Appelman HD. Solitary solid stromal gastrointestinal tumors in von Recklinghausen’s disease with minimal smooth muscle differentiation. Hum Pathol 1984; 15: 229-32.
4) Walsh NMG, Bodhurtha A. Auerbach’s myenteric plexus: a possible site of origin for gastrointestinal stromal tumors in von Recklinghausen’s disease. Arch Pathol Lab Med 1990; 114: 522-5.
5) Miettinen M, Sarlomo-Rikala M, Lasota J. Gastrointestinal stromal tumors: recent advances in understanding of their biology. Hum Pathol 1999; 30: 1213-20.[PubMed]
6) Mori K, Chiba N, Yamamoto Y, Yonekawa H. A case of perforated gastrointestinal stromal tumor of the ileum accompanied with von Recklinghausen’s disease (in Japanese with English abstract) . Jpn Clin Surg 2002; 63: 2980-4.
7) Guily JA, Picand R, Giuly D, Monges B, Nguyen-Cat R. Von Recklinghausen disease and gastrointestinal stromal tumors. Am J Surg 2002; 185: 86-7.
8) Takazawa Y, Sakurai S, Sakuma Y, Ikeda T, Yamaguchi J, Hashizume Y, Yokoyama S, Motegi A, Fukayama M. Gastrointestinal stromal tumors of neurofibromatosis type I (von Recklinghausen’s disease) . Am J Surg Pathol 2005; 29: 755-63.[PubMed]
9) Anderson J, Sihto H, Meis-Kindblom JM, Joensuu H, Nupponen N, Kindblom LG. NF1-associated gastrointestinal stromal tumors have unique clinical, phenotypic, and genotypic characteristics. Am J Surg Pathol 2005; 29: 1170-6.
10) Miettinen M, Fetsch JF, Sobin LH, Lasota J. Gastrointestinal stromal tumors in patients with neurofibromatosis 1: a clinicopathologic and molecular genetic study of 45 cases. Am J Surg Pathol 2006; 30: 90-6.
11) Li Y, Bollag G, Clark R, Stevens J, Conroy L, Fults D, Ward K, Friedman E, Samowitz W, Robertson M, Bradley P, McCormick F, White R, Cawthon R. Somatic mutations in the neurofibromatosis type 1 gene in human tumors. Cell 1992; 69: 275-81.
12) Bollag G, McCormick F. Differential regulation of rasGAP and neurofibromatosis gene product activities. Nature 1991; 351: 576-9.[PubMed]
13) Kim HR, Kim YJ. Neurofibromatosis of the colon and rectum combined with other manifestations of von Recklinghausen’s disease: report of a case. Dis Colon Rectum 1998; 41: 1187-92. [PubMed] |
|
Others
1) Division of Surgery, Kuniyoshi Hospital, Isumi 298-0123.
2) Department of General Surgery and 3) Frontier Surgery, Graduate School of Medicine, Chiba University, Chiba 260-8670.
和城光庸1,2),小田健司2),大嶋博一1),大森敏生1),姫野雄司1),清家和裕2),幸田圭史3),宮崎 勝2): 神経線維腫症1型に生じた胃GISTおよびS状結腸神経線維腫の1例.
1) 国保国吉病院外科
2) 千葉大学大学院医学研究院臓器制御外科学
3) 千葉大学大学院医学研究院先端応用外科学
Tel. 043-222-7171. Fax. 043-226-2552. e-mail: odaken-cib@umin.ac.jp
2006年3月7日受付,2006年5月15日受理.
|
|
|
|
|
|
|
|
|
|